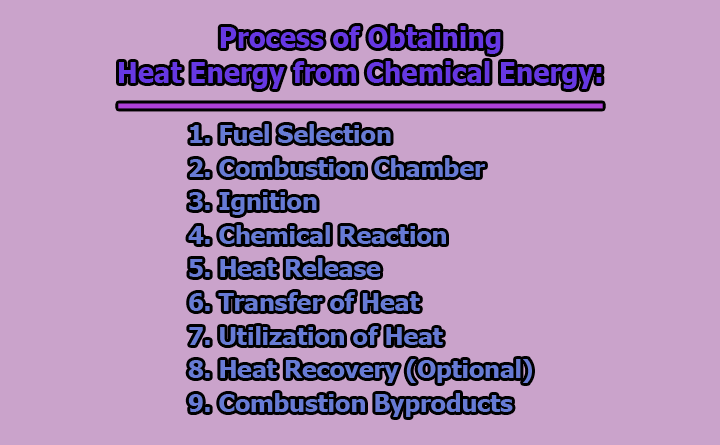
Process of Obtaining Heat Energy from Chemical Energy:
Obtaining heat energy from chemical energy involves the process of combustion, wherein a chemical reaction between a fuel and an oxidizing agent releases heat. This process is fundamental in various applications, from powering vehicles to generating electricity. Let’s explore the process of obtaining heat energy from chemical energy:
1. Fuel Selection: The first step in obtaining heat energy from chemical energy involves the careful selection of an appropriate fuel. Fuels are substances that can undergo combustion reactions, releasing energy in the form of heat. Common fuels include hydrocarbons like gasoline, diesel, natural gas, and solid fuels like coal or biomass. The choice of fuel depends on factors such as energy content, availability, and environmental considerations. For instance, in internal combustion engines, liquid fuels like gasoline or diesel are often chosen for their high energy density, while in power plants, coal or natural gas might be preferred based on economic and efficiency considerations.
2. Combustion Chamber: Once the fuel is selected, it is introduced into a controlled environment known as the combustion chamber. This chamber is designed to facilitate and regulate the combustion reaction. It provides a confined space where the fuel can mix with an oxidizing agent, typically atmospheric oxygen (O2). The controlled environment ensures safety and allows for optimal combustion conditions. Combustion chambers are integral components of various systems, including internal combustion engines, industrial furnaces, and power plant boilers.
3. Ignition: Ignition is the process of initiating the combustion reaction by providing an external energy source. This energy input is necessary to break the initial chemical bonds within the fuel molecules, initiating the reaction. In internal combustion engines, spark plugs generate sparks to ignite the air-fuel mixture. In industrial furnaces or power plants, ignition may involve pilot lights, electric igniters, or other methods. The timing and efficiency of ignition are crucial for controlling the combustion process and ensuring the release of heat at the desired moment.
4. Chemical Reaction: The heart of obtaining heat energy from chemical energy lies in the combustion reaction itself. This reaction involves the combination of the fuel with oxygen from the air. For hydrocarbon fuels like gasoline, the general combustion reaction can be represented as follows:
Fuel + Oxygen → Carbon Dioxide + Water + Heat
The combustion process is exothermic, meaning it releases energy in the form of heat and light. The chemical bonds within the fuel molecules are broken, and new bonds are formed in the resulting combustion byproducts.
5. Heat Release: As the combustion reaction progresses, the heat generated increases the temperature of the gases in the combustion chamber. This rise in temperature is a direct result of the energy released during the exothermic combustion reaction. The heat energy is then available for transfer to a working fluid or the desired medium in the system. The efficiency of this heat release is crucial for optimizing the overall performance of the system, whether it be a car engine, a power plant, or a heating system in a residential setting.
6. Transfer of Heat: Following the release of heat in the combustion chamber, the next step involves the transfer of this thermal energy to a working fluid or directly to the medium requiring heating. In internal combustion engines, the heat is transferred to the engine components and the working fluid (air-fuel mixture), leading to an increase in temperature and pressure. In power plants, the heat is transferred to water to produce steam, which is then directed to turbines for electricity generation. The efficiency of this heat transfer process is critical for maximizing the useful work output of the system.
7. Utilization of Heat: Once the heat is transferred, it is harnessed for specific applications. The utilization of heat energy depends on the design and purpose of the system. For examples:
- In internal combustion engines, the heat energy is converted into mechanical work through the expansion of gases in the engine cylinder, ultimately propelling the vehicle.
- In power plants, the heat energy is used to produce steam, and the high-pressure steam drives turbines connected to generators, generating electricity for various applications.
- In residential heating systems, the transferred heat is distributed to living spaces, ensuring a comfortable indoor environment.
8. Heat Recovery (Optional): In certain systems, there is a focus on recovering and reusing excess heat that might otherwise be wasted. Heat recovery systems, such as heat exchangers, are employed to capture and transfer heat from one part of the system to another. This process improves overall efficiency by utilizing heat that would otherwise be lost to the surroundings. Heat recovery is particularly relevant in industrial processes and power generation, where optimizing energy use is a key consideration.
9. Combustion Byproducts: Alongside the release of heat, the combustion process generates byproducts, including carbon dioxide (CO2), water vapor (H2O), and potentially other pollutants depending on the fuel composition. Managing and minimizing the environmental impact of these byproducts is essential. Modern technologies, such as catalytic converters and exhaust treatment systems, aim to reduce harmful emissions and enhance the environmental sustainability of combustion-based processes. Understanding and controlling the production of combustion byproducts is crucial for meeting environmental regulations and promoting cleaner energy practices.
In conclusion, obtaining heat energy from chemical energy is a complex process involving the careful selection of fuels, controlled combustion reactions, and the efficient transfer and utilization of the released heat. This process underlies various essential applications across industries and everyday life, contributing to power generation, transportation, heating, and more.

Assistant Teacher at Zinzira Pir Mohammad Pilot School and College